We are happy to announce that ANTI-SUPERBUGS PCP, in collaboration with CORDIS, has published a Results in Brief article on the CORDIS website in six languages.
The article is available here
We are happy to announce that ANTI-SUPERBUGS PCP, in collaboration with CORDIS, has published a Results in Brief article on the CORDIS website in six languages.
The article is available here
ANTI-SUPERBUGS PCP was presented in the EHMA 2022 Annual Conference last Thursday June 16th in Brussels (Belgium).
Representing the project Consortium, Enric Limon (VINCat/ICO) presented the poster which was titled as “ANTI-SUPERBUGS PCP. A cross-border joint action to improve healthcare-associated infections’ prevention and control systems”
ANTI-SUPERBUGS PCP was presented in the 32nd European Congress of Clinical Microbiology & Infectious Diseases last Tuesday April 26th in Lisbon (Portugal).
Representing the project Consortium, Ion Arrizabalaga (AQuAS) orally presented obtained results under the Healthcare-associated infections, infection prevention & control section.
Do not lose the chance to watch the details of ANTI-SUPERBUGS PCP Final Event, which took place remotely on Wednesday March 30th 2022.
The project is presenting its achieved results in a public remote event on Wednesday March 30th via Zoom.
The event will count on Buyers Group’s insights, Bugwatcher and Sens4Care contractors’ innovative solution demonstration as well as key-note speakers’ participation.
Don’t lose the chance to see how Pre-Commercial Procurement procedure has been implemented as a tool to foster innovation and fight Antimicrobial Resistance!
You can already register in the online form available here
More details will be presented soon…
The ANTI-SUPERBUGS PCP project is presenting its results in the 32nd European Congress of Clinical Microbiology & Infectious Diseases, taking place in Lisbon on April 23-26 2022.
It will orally present its results during the Interventions to prevent HAIs: best learning experiences session. Moreover, it will be presented as a Poster under the Hospital epidemiology, transmission, surveillance & screening session.
In the same manner, ANTI-SUPERBUGS PCP is presenting its results in the XLIX National Congress of the Italian Association of Clinical Microbiologists (AMCLI), taking place in Rimini between February 26th and March 1st 2022.
Both Consortium and Phase 3 final contractors (Bugwatcher and Sens4Care) are testing the capability of their prototypes to detect the presence of MROs in the real context at the facilities of HELIOS (Germany), Provincia autonoma di Trento (Italy) and Fundació Assistencial Mútua de Terrassa (Spain).
More information coming…
Meanwhile, have a look at the presented abstracts by final contractors at the beginning of Phase 3: Bugwatcher and Sens4Care.
All three contractors (Bugwatcher, Culture, Sens4Care) successfully executed Phase 2 contracts by developing prototypes that demonstrated their capacity to cover the common identified unmet need of non-invasively detect the presence of multi-resistant organisms (MROs) in lab conditions.
The three of them will be invited to present their offers to participate in Phase 3 and deploy their first set of prototypes to prove their ability to detect the presence of MROs in the real context at the facilities of HELIOS (Germany), Provincia autonoma di Trento (Italy) and Fundació Assistencial Mútua de Terrassa (Spain).
Have a look at the summary of main results achieved by all three participating contractors in Phase 2: Bugwatcher; Culture; Sens4Care
Three active contractors (Sens4Care, Culture and Bugwatcher) continue working in Phase 2 – Prototyping tasks, which will end by March 22nd 2021. Stay tuned as main results achieved by the contractors in this phase 2 will be published in the website.
Meanwhile, have a look at Phase 1 already achieved results by all four participating contractors: CULTURE ; Sens4Care ; BUGWATCHER ; ASB-IMS²
On the 7th of September 2020 there has been the kick-off meeting of Phase II.
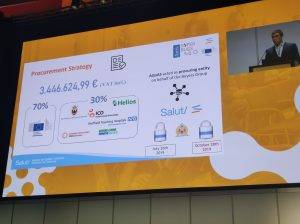
On the 31st of July 2020, Anti-SUPERBugs PCP has the awarding decision of Phase II “Prototyping”. Details of the award and documentation available here.
This website uses 'cookies' to give you the best, most relevant experience. Using this website we'll assume you're ok with this. You can opt-out at any time if you wish. To find out more about cookies, see our 'Privacy and Cookie Policy' page
The cookie settings on this website are set to "allow cookies" to give you the best browsing experience possible. If you continue to use this website without changing your cookie settings or you click "Accept" below then you are consenting to this.